
“My hair was thinning, but now it’s thick almost like it was when I was younger. I’m totally sold on TrichoCyte.”

Treatment
Day 0
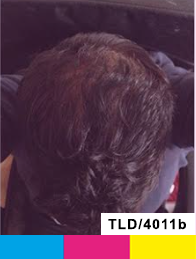
Treatment
Day 90
Study Subject #1
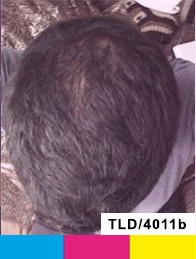
Treatment
Day 180

Treatment
Day 270
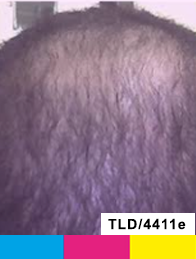
Treatment
Day 0

Treatment
Day 90
Study Subject #2

Treatment
Day 180
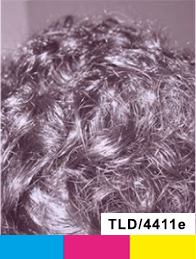
Treatment
Day 270
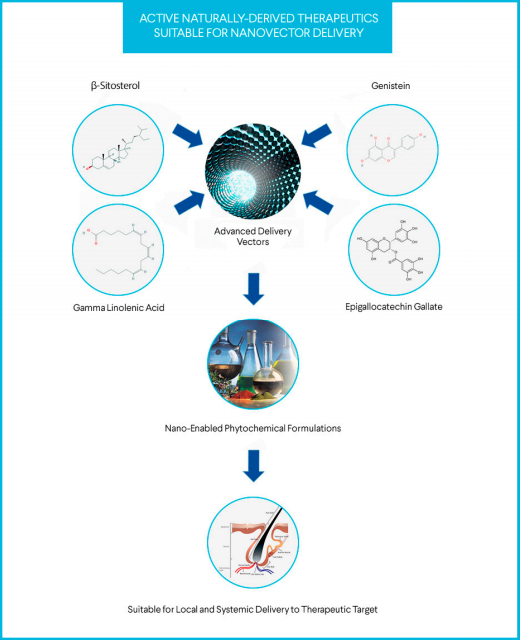
Enhanced Drug Delivery Of Natural
Hair Stimulating Compounds
In a landmark peer-reviewed study, the authors Geno Marcovici, Ph.D., DABAAHP and Alan Bauman, M.D. demonstrated an amplified clinical outcome for a proprietary formulation incorporating naturally-based anti-inflammatory compounds & highly potent 5 alpha-reductase inhibitors. The study described the enhanced outcome resulting via the successful invagination of the active formula in a highly efficient drug-delivery vector molecule, beta-cyclodextrin. This study appears to report the first use of naturally-based drug-delivery vectors as agonists in amplifying the clinical benefit of botanically-based hair growth formulations.
 An Uncontrolled Case Series Using a Botanically Derived, β-Cyclodextrin Inclusion Complex in Two Androgenetic Alopecia-Affected Male Subjects †
An Uncontrolled Case Series Using a Botanically Derived, β-Cyclodextrin Inclusion Complex in Two Androgenetic Alopecia-Affected Male Subjects †
Geno Marcovici 1,* and Alan Bauman 2
Received: 13 July 2020; Accepted: 5 August 2020; Published: 10 August 2020
ART Inc., 101 Raintree Ln, Mahwah, NJ 07430, USA
Bauman Medical Center, 1450 S. Dixie Hwy, Boca Raton, FL 33432, USA; [email protected] *
Correspondence: [email protected];
† Abbreviated Title: Nanotechnology and botanical hair growth formulations.
Abstract: Drug-based monotherapy provides limited clinical benefits in polygenic disorders, such as androgenetic alopecia. Possible benefits must be measured against non-trivial risks of negative side effects. Several well-controlled, peer-reviewed, basic science studies have demonstrated novel mechanisms of action and potential utility for natural-based phytochemicals in the treatment of androgen-mediated disorders, including androgenetic alopecia. Yet, due to phytochemical instability, volatility, and incompatibility, the bridge from in vitro potential to clinical efficacy remains largely unmet. Recent advances in nanomaterial manipulation provide enhanced platforms, such as cyclodextrins, in which these phytochemicals may be enveloped and delivered without triggering the loss of intended function. Unexpected, positive results of an uncontrolled case series for a cyclodextrin-enabled, natural-based formula containing γ linolenic acid, β-Sitosterol, epigallocatechin gallate, and genistein, administered concomitantly via oral and topical form in two androgenetic alopecia-affected, male subjects over the course of 270 days were found. At baseline, significant baldness in the vertex scalp of both subjects was observed. Subsequent 90-day time points demonstrated marked hair thickening. On treatment day 270 (conclusion), scalp hair loss was no longer evident in either patient. Particularly in the setting of a disorders, such as androgenetic alopecia, nano-complexed, botanically-based compositions may offer beneficial adjunctives or alternatives to traditional drug-based/surgical medical treatments.
Keywords: hair loss; advanced drug delivery; nanoparticles; natural hair loss treatment; phytochemicals;
β-cyclodextrin
Beta Cyclodextrin
β-Cyclodextrin (β-CD) is a cone-shaped molecule. Hydrophilic at the outer surface of the cavity for many hydroxyl groups, but hydrophobic in the cavity. β-Cyclodextrin is a cyclic, nontoxic oligosaccharide consisting of seven glucose units joined as α-(1 → 4) isomers. With a cavity at the center of the molecular arrangement β-CD can thus form a stable insoluble inclusion complex with numerous naturally-based active chemicals, including β-sitosterol. Further, as β-CD is soluble in water, a variety of hydrophobic guest molecules can be encapsulated in its non-polar cavity. Such characteristics are now being successfully tested and applied in the realms of drug-controlled release, separation and adsorption.
“My hairstylist said “what have you been doing, your hair is so much thicker”
“Can’t say enough about how impressed I am by this new product. It’s better than any hair drug I tried, for sure. And I love that it’s safe and natural.”
Functional Hypothesis Supported with
20+ Years of Critically Peer-Reviewed,
Published Medical Research
A randomized, double-blind, placebo-controlled trial to determine the effectiveness of botanically derived inhibitors of 5-alpha-reductase in the treatment of androgenetic alopecia
Nelson Prager 1, Karen Bickett, Nita French, Geno Marcovici
- PMID: 12006122 DOI: 10.1089/acm.2002.8.143
- J Altern Complement Med. 2006 Mar;12(2):199
Abstract
Background: Androgenetic alopecia (AGA) is characterized by the structural miniaturization of androgen-sensitive hair follicles in susceptible individuals and is anatomically defined within a given pattern of the scalp. Biochemically, one contributing factor of this disorder is the conversion of testosterone (T) to dihydrotestosterone (DHT) via the enzyme 5-alpha reductase (5AR). This metabolism is also key to the onset and progression of benign prostatic hyperplasia (BPH). Furthermore, AGA has also been shown to be responsive to drugs and agents used to treat BPH. Of note, certain botanical compounds have previously demonstrated efficacy against BPH. Here, we report the first example of a placebo-controlled, double-blind study undertaken in order to examine the benefit of these botanical substances in the treatment of AGA.
Objectives: The goal of this study was to test botanically derived 5AR inhibitors, specifically the liposterolic extract of Serenoa repens (LSESr) and beta-sitosterol, in the treatment of AGA.
Subjects: Included in this study were males between the ages of 23 and 64 years of age, in good health, with mild to moderate AGA.
Results: The results of this pilot study showed a highly positive response to treatment. The blinded investigative staff assessment report showed that 60% of (6/10) study subjects dosed with the active study formulation were rated as improved at the final visit.
Conclusions: This study establishes the effectiveness of naturally occurring 5AR inhibitors against AGA for the first time, and justifies the expansion to larger trials.
Inhibition of inflammatory gene expression in keratinocytes using a composition containing Carnitine, Thioctic Acid & LSESr
Sridar Chittur 1, Brian Parr, Geno Marcovici
- PMID: 19692448 PMCID: PMC3137880 DOI: 10.1093/ecam/nep102
Abstract
Chronic inflammation of the hair follicle (HF) is considered a contributing factor in the pathogenesis of androgenetic alopecia (AGA). Previously, we clinically tested liposterolic extract of Serenoa repens (LSESr) and its glycoside, β-sitosterol, in subjects with AGA and showed a highly positive response to treatment. In this study, we sought to determine whether blockade of inflammation using a composition containing LSESr as well as two anti-inflammatory agents (carnitine and thioctic acid) could alter the expression of molecular markers of inflammation in a well-established in vitro system. Using a well-validated assay representative of HF keratinocytes, specifically, stimulation of cultured human keratinocyte cells in vitro, we measured changes in gene expression of a spectrum of well-known inflammatory markers. Lipopolysaccharide (LPS) provided an inflammatory stimulus. In particular, we found that the composition effectively suppressed LPS-activated gene expression of chemokines, including CCL17, CXCL6 and LTB(4) associated with pathways involved in inflammation and apoptosis. Our data support the hypothesis that the test compound exhibits anti-inflammatory characteristics in a well-established in vitro assay representing HF keratinocyte gene expression. These findings suggest that 5-alpha reductase inhibitors combined with blockade of inflammatory processes could represent a novel two-pronged approach in the treatment of AGA with improved efficacy over current modalities.
Blockade of Androgen Markers Using a Novel Betasitosterol, Thioctic Acid and Carnitine-containing Compound in Prostate and Hair Follicle Cell-based Assays
Li Chen 1, Jiaolong Wang 1, Glen Mouser 2, Yan Chun Li 1, Geno Marcovici 3
- PMID: 26990224 DOI: 10.1002/ptr.5611
Abstract
Androgenetic alopecia (AGA) affects approximately 70% of men and 40% of women in an age-dependent manner and is partially mediated by androgen hormones. Benign prostatic hyperplasia (BPH) similarly affects 50% of the male population, rising by 10% each decade. Finasteride inhibits 5-alpha reductase (5AR) and is used to treat both disorders, despite offering limited clinical benefits accompanied by significant adverse side effects. Building on our previous work demonstrating the efficacy of naturally derived 5AR inhibitors (such as stigmasterol and beta sitosterol), we hypothesize that targeting 5AR as well as inflammatory pathways may yield improved efficacy in AGA and BPH. Here we address these dual pathomechanisms by examining the potency of a novel composition using in vitro assays of representative cell lines for AGA (hair follicle dermal papilla cells) and BPH (LNCaP prostate cells), respectively. Exposure of cells to the novel test composition down-regulated mRNA expression profiles characteristic of both disease processes, which outperformed finasteride. Changes in mRNA expression were corroborated at the protein level as assessed by western blotting. These studies provide proof of concept that novel, naturally derived compositions simultaneously targeting 5AR and inflammatory mediators may represent a rational approach to treating AGA and BPH. Copyright © 2016 John Wiley & Sons, Ltd.
Keywords: 5-alpha reductase; androgenetic alopecia; botanicals; phytotherapy.
ONGOING R&D KEEPS YOUR PRACTICE ON THE CUTTING EDGE OF TECHNOLOGY IN THE FIELD
We offer the latest, greatest developments arising from research conducted in our facilities as well as those of our collaborative partners at University of Chicago, SUNY, New York and elsewhere. Furthermore, our R&D is backed by numerous published studies – found in the peer-reviewed medical and scientific literature. What other naturally-based hair loss armamentarium can make this claim?

ACCESS FULL MARKETING MATERIALS
We take the time to understand your marketing requirements and aim to provide you with a selection of tailored marketing tools and support to ensure the success of the TRICHOCYTE™ brand in each clinical practice.

DEDICATED SENIOR CONTACT
As an authorized distributor you’ll have direct contact with a dedicated and personalized contact in our company. This means that you’ll be communicating with experts and decision makers who can help grow the positive business success the TRICHOCYTE™ brand represents to your company.
OUR EXPERTISE
As we manufacture only the highest quality healthcare products we have established success within multiple sectors in numerous distribution channels including: Supply and dissemination of our proprietary formulations to pharmacies, grocery sector and hospitals, as well as working with governments and healthcare tenders.

CREATING A RANGE THAT WORKS FOR YOU AND YOUR PATIENT BASE
At TRICHOCYTE™, we understand that some haircare products are not suitable for every practice. This is why we take the time to understand your needs and requirements so as to create and tailor products specific to your practice needs.

cGMP / QUALITY CONTROL
All TRICHOCYTE™ product formulations are manufactured under tight cGMP standards with state of the art QC and QA in place. This, to ensure that all formulations produced by us meet the highest possible safety, efficacy, purity, and stability standards. Our compliance with cGMP regulations is further verified by reviewing the company’s documented adherence. Because random regulatory inspections of commercial laboratories can be conducted anytime—without prior notification—it has long been our practice to conduct routine comprehensive cGMP self-evaluations. Finally, all skus and LOTS are assigned critical batch numbers thereby providing you the assurance that you’ll always be in possession of the very finest hair treatment formulas available anywhere.

Contact Us
(For Distribution Opportunities, Pricing & Ordering)